Notebooks
Premium
Trends
BioTuring
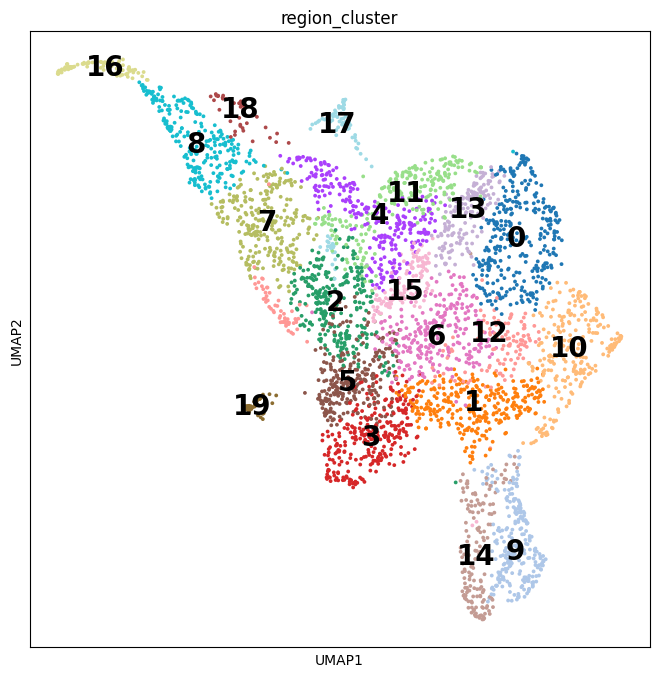
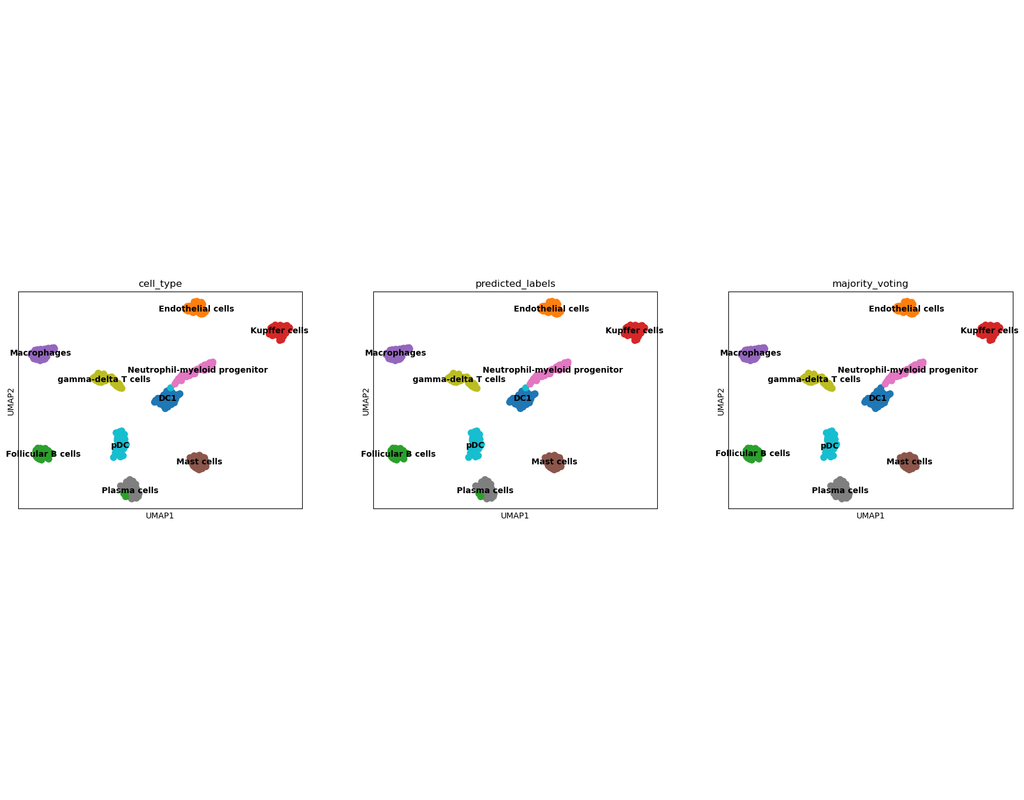
CellTypist is an automated cell type annotation tool for scRNA-seq datasets on the basis of logistic regression classifiers optimised by the stochastic gradient descent algorithm. CellTypist allows for cell prediction using either built-in (with a current focus on immune sub-populations)or custom models, in order to assist in the accurate classification of different cell types and subtypes.
CellTypist can identify 101 cell types or states from more than one million cells, including previously underappreciated cell states.
For the CellTypist pre-trained models, immune cells from 20 tissues of 19 studies were collected and harmonized into consistent labels. These cells were split into equal-sized mini-batches, and these batches were sequentially trained by the l2-regularized logistic regression using stochastic gradient descent learning. Feature selection was performed to choose the top 300 genes from each cell type, and the union of these genes was supplied as the input for a second round of training.