Notebooks
Premium
Trends

BioTuring
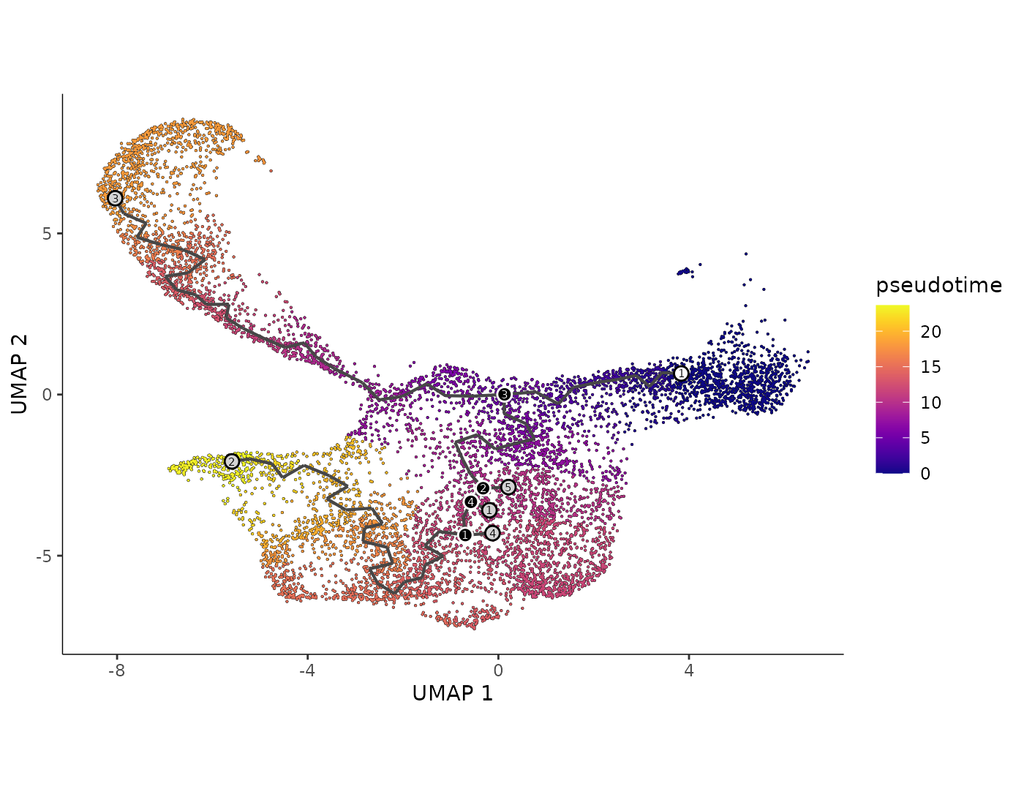
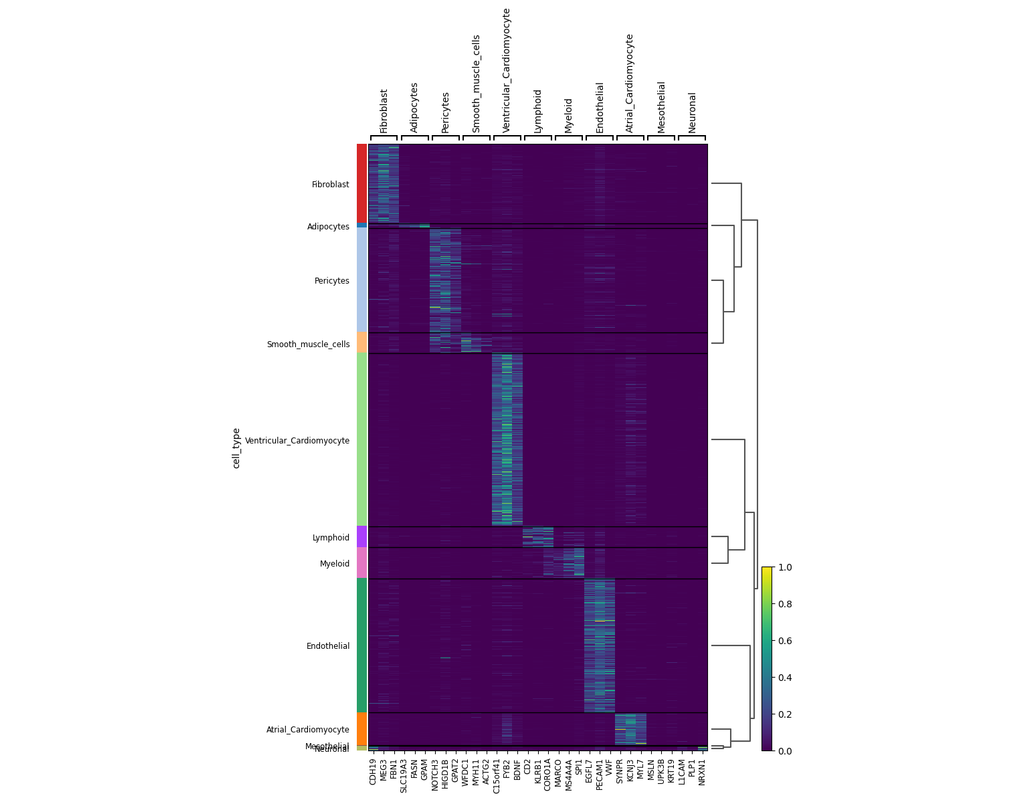
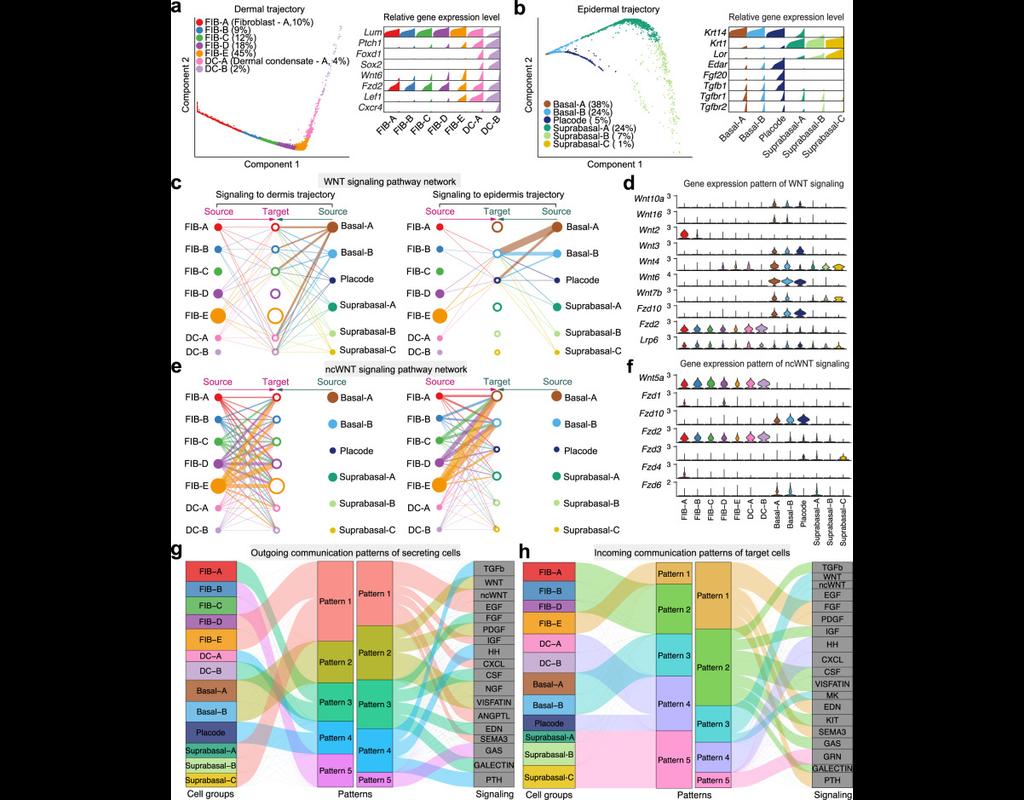
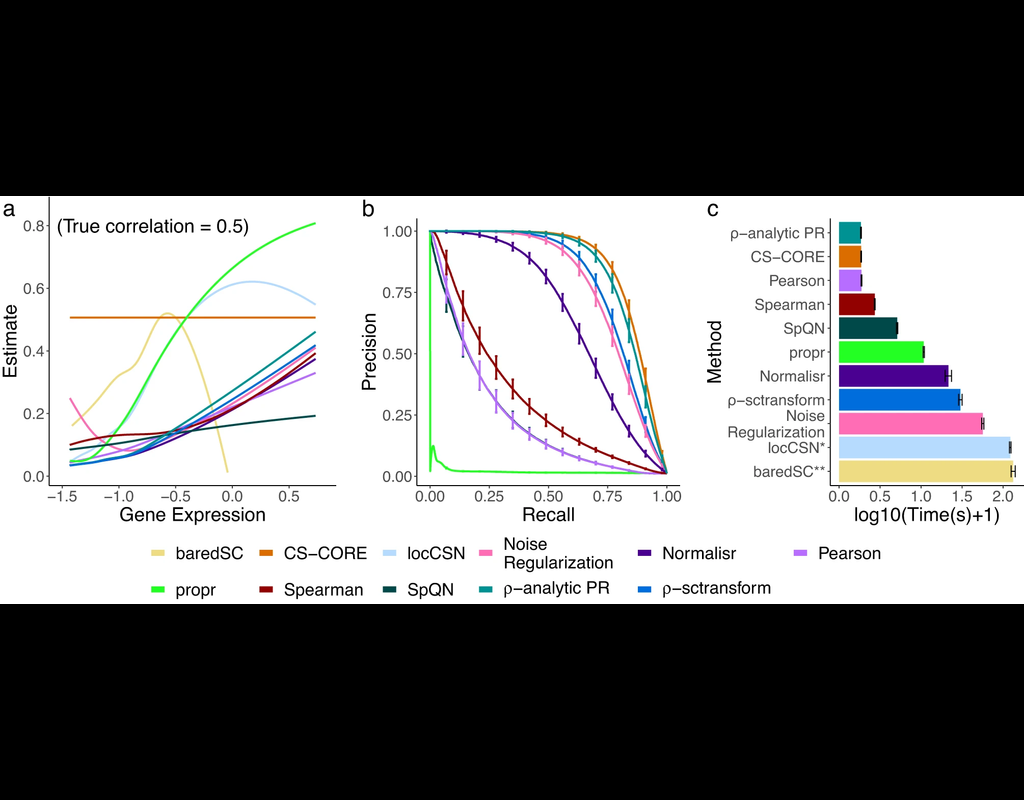
Single-cell data analysis is revolutionizing biological research, but often these dataset sizes can be massive and pose challenges for submission process. Bioalpha-Biocolab addresses this issue by implementing advanced algorithms and leveraging efficient computational resources to overcome these challenges.